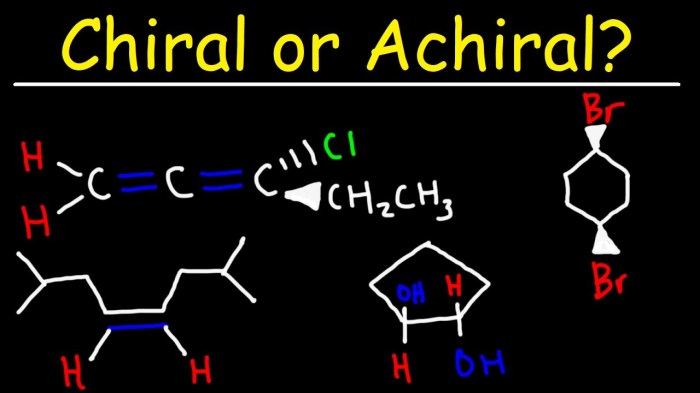
Is the molecule shown below chiral or achiral – In the realm of chemistry, chirality emerges as a captivating concept that profoundly influences the behavior and properties of molecules. This discourse embarks on an exploration of chirality, unraveling its intricate relationship with molecular symmetry, optical activity, and biological significance.
Chirality, the asymmetry of molecules, stems from the absence of a superimposable mirror image. Understanding chirality is paramount in comprehending the behavior of molecules in various contexts, ranging from drug design to enzyme-substrate interactions.
Molecular Chirality
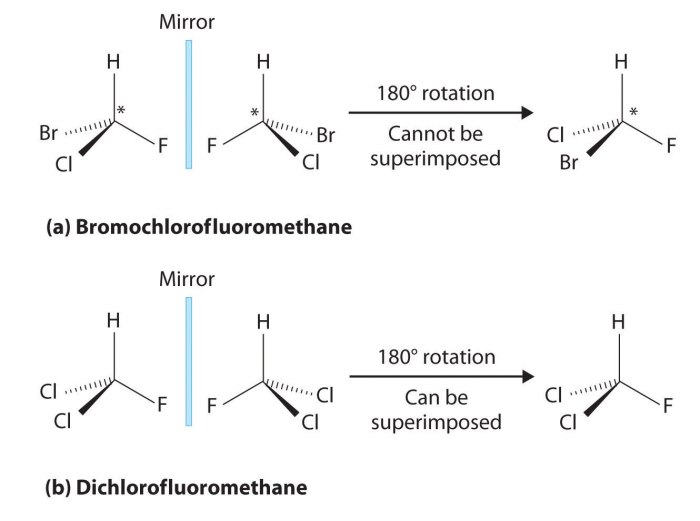
Chirality is a fundamental property of molecules that plays a crucial role in their interactions and biological functions. It refers to the lack of symmetry in a molecule, resulting in the existence of non-superimposable mirror images.
Structural Symmetry
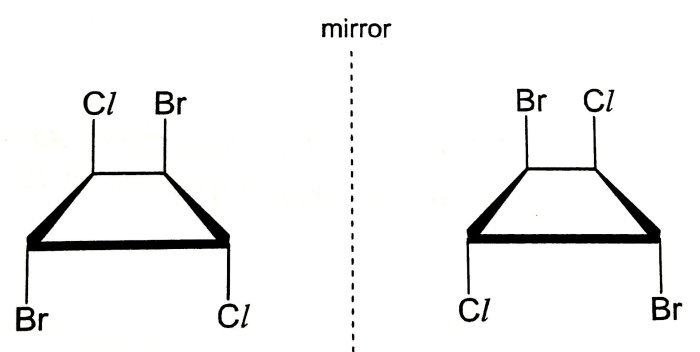
Molecular symmetry is the arrangement of atoms or groups of atoms in a molecule that creates a balanced and symmetrical structure. Symmetry elements such as planes, axes, and centers can help determine the chirality of a molecule.
- Plane of Symmetry:A plane that divides a molecule into two mirror-image halves.
- Axis of Symmetry:A line that passes through the molecule and divides it into two identical halves.
- Center of Symmetry:A point in the molecule that is equidistant from all atoms.
Chiral Centers
Chiral centers are atoms that are bonded to four different groups or atoms. The presence of a chiral center makes a molecule chiral.
To identify chiral centers, check if an atom has four different groups attached to it. If so, the atom is a chiral center, and the molecule is chiral.
Optical Activity
Optical activity is the ability of a chiral molecule to rotate plane-polarized light. The direction and extent of rotation depend on the specific chiral molecule.
Molecules that rotate plane-polarized light clockwise are designated as dextrorotatory (+), while those that rotate it counterclockwise are levorotatory (-).
Molecular Handedness
Molecular handedness refers to the existence of two non-superimposable mirror-image forms of a chiral molecule. These forms are called enantiomers.
Enantiomers have the same chemical formula and connectivity but differ in their spatial arrangement. They are like left- and right-handed versions of the same molecule.
Stereochemistry
Stereochemistry is the study of the three-dimensional arrangement of atoms and groups of atoms in molecules.
Stereoisomers are molecules with the same molecular formula but different spatial arrangements. Enantiomers and diastereomers are two types of stereoisomers.
- Enantiomers:Non-superimposable mirror images of each other.
- Diastereomers:Stereoisomers that are not mirror images of each other.
Chirality in Biological Systems, Is the molecule shown below chiral or achiral
Chirality plays a crucial role in biological processes, including enzyme-substrate interactions and drug design.
Enzymes are proteins that catalyze specific chemical reactions. The chirality of an enzyme’s active site determines which enantiomer of a substrate it will bind to.
In drug design, the chirality of a drug molecule can affect its efficacy and toxicity.
Clarifying Questions: Is The Molecule Shown Below Chiral Or Achiral
What is the significance of chirality in biological systems?
Chirality plays a crucial role in biological processes, as many molecules in living organisms, such as amino acids and sugars, exist in chiral forms. The specific chirality of these molecules is essential for their biological functions, including enzyme recognition and receptor binding.
How can optical activity be used to determine chirality?
Optical activity refers to the ability of a chiral molecule to rotate plane-polarized light. The direction and extent of rotation can be used to determine the chirality of the molecule. Chiral molecules that rotate light clockwise are designated as (+)-enantiomers, while those that rotate light counterclockwise are designated as (-)-enantiomers.
What is the difference between enantiomers and diastereomers?
Enantiomers are pairs of chiral molecules that are mirror images of each other. Diastereomers, on the other hand, are stereoisomers that are not mirror images. Diastereomers have different physical and chemical properties, unlike enantiomers, which have identical physical properties but opposite optical activities.