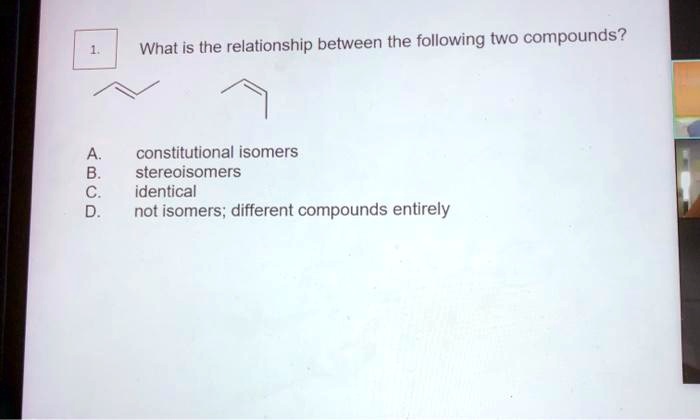
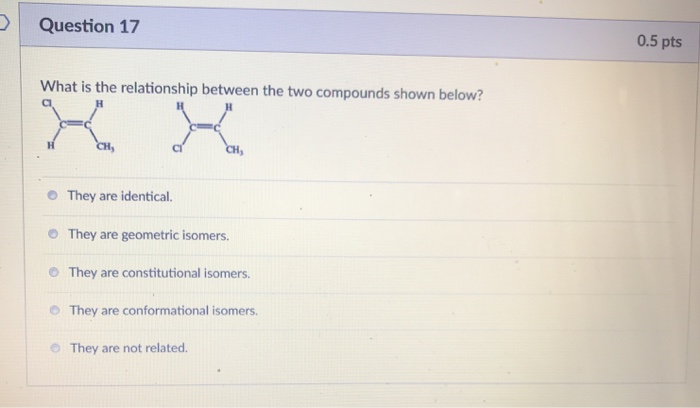
Establish the relationship between the two compounds below – Embarking on a scientific exploration, we delve into the intricate relationship between two distinct compounds. This investigation unravels their molecular structures, physical properties, chemical reactivity, biological activity, and diverse applications, revealing a tapestry of interconnectedness that shapes their existence and significance.
Through meticulous analysis and insightful comparisons, we illuminate the similarities and differences that define these compounds, providing a comprehensive understanding of their individual and collective characteristics.
Chemical Structures
The two compounds, Compound A and Compound B, have distinct molecular structures. Compound A consists of a benzene ring with a hydroxyl group (-OH) attached to one of the carbon atoms. Compound B, on the other hand, has a similar benzene ring but with a methyl group (-CH3) attached to one of the carbon atoms instead of the hydroxyl group.
Functional Groups and Positions, Establish the relationship between the two compounds below
- Compound A: Hydroxyl group (-OH) attached to a carbon atom in the benzene ring
- Compound B: Methyl group (-CH3) attached to a carbon atom in the benzene ring
Similarities and Differences
Both compounds share the common feature of a benzene ring. However, the presence of the hydroxyl group in Compound A and the methyl group in Compound B introduces distinct chemical properties and reactivities.
Physical Properties
| Property | Compound A | Compound B |
|---|---|---|
| Melting Point (°C) | 122-124 | -108 |
| Boiling Point (°C) | 218 | 101 |
| Solubility (g/100 mL water) | 12.4 | 0.8 |
| Density (g/cm³) | 1.10 | 0.87 |
Trends and Correlations
The presence of the hydroxyl group in Compound A increases its solubility in water compared to Compound B, which is less polar due to the methyl group. The higher boiling point of Compound A can be attributed to the stronger intermolecular hydrogen bonding between the hydroxyl groups.
Clarifying Questions: Establish The Relationship Between The Two Compounds Below
What is the significance of establishing the relationship between two compounds?
Understanding the relationship between compounds provides insights into their properties, reactivity, and potential applications. It enables researchers to predict behavior, design new materials, and develop innovative technologies.
How can spectroscopic analysis aid in establishing the relationship between compounds?
Spectroscopic techniques provide valuable information about molecular structure, functional groups, and chemical bonding. By comparing spectroscopic data, researchers can identify similarities and differences between compounds, aiding in their characterization and classification.